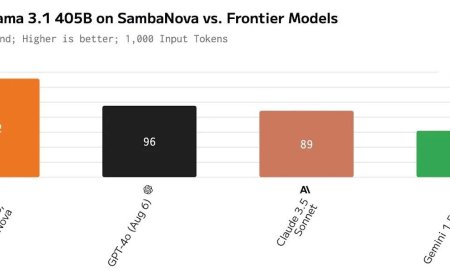
U.S. FDA authorizes first OTC hearing aid software to be used with Apple’s AirPods Pro 2
The U.S. Food and Drug Administration (FDA) on Thursday authorized the first over-the-counter hearing aid software that is intended to be… The post U.S. FDA authorizes first OTC hearing aid software to be used with Apple’s AirPods Pro 2 appeared first on MacDailyNews.
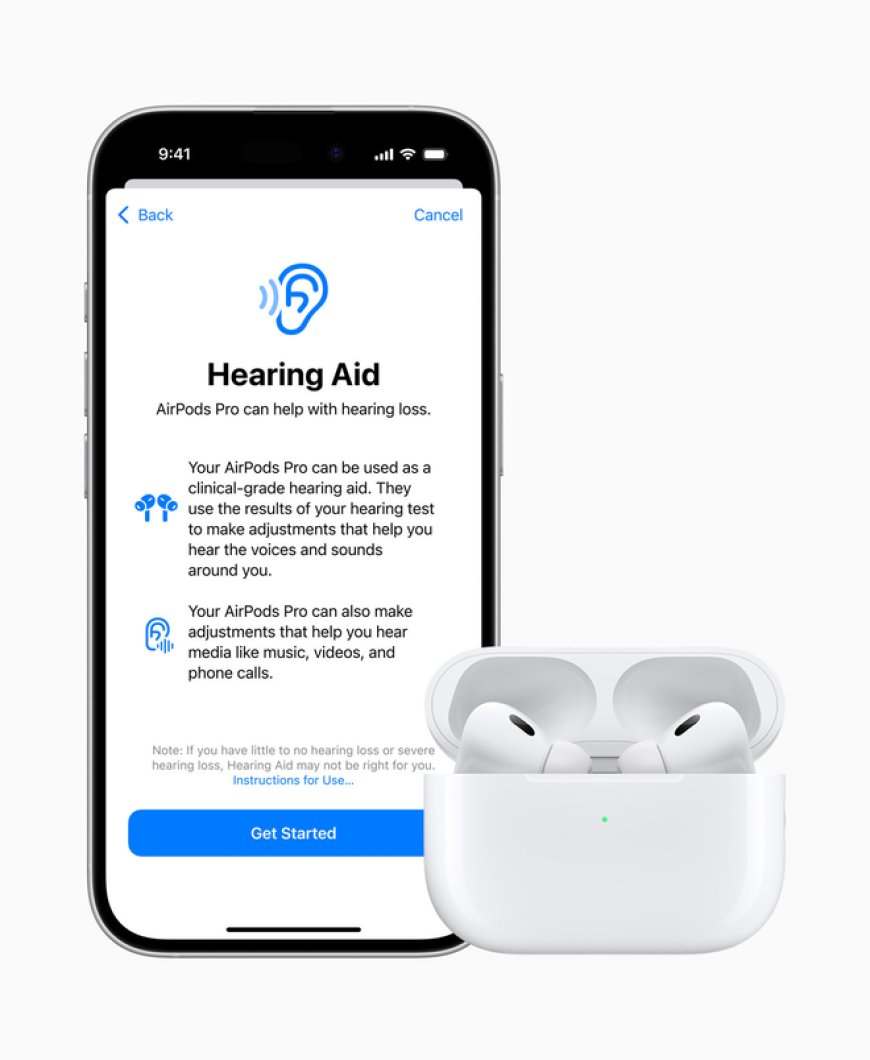

The U.S. Food and Drug Administration (FDA) on Thursday authorized the first over-the-counter hearing aid software that is intended to be used with compatible versions of the Apple AirPods Pro headphones.
Reuters:
New hearing health features will be available this fall for AirPods Pro 2 users in more than 100 countries and regions, Apple had said.
Once installed and customized to the user’s hearing needs, the software enables versions of the AirPods Pro to serve as an OTC hearing aid, that is intended to amplify sounds for 18 years or older people with perceived mild-to-moderate hearing impairment, the health regulator said.
Apple’s Hearing Aid Feature, authorized on Thursday, is a software-only mobile medical application and is set up using an iOS device like an iPhone and the user’s hearing levels are accessed from the iOS HealthKit to customize the feature.
What's Your Reaction?